What is brass and where is it used?

Brass is an industrial alloy that allows you to reduce the cost of manufacturing products that use non-ferrous metals. Compared to pure copper, brass products are several times cheaper.



Properties
Brass looks like a yellowish white metallic alloy. It is a bit like bronze in color, since one of its components is copper. Zinc is used as the second base metal. But the characteristics of bronze and brass differ significantly.
Heating the alloy affects the structural change. As the temperature rises, the zinc and copper atoms are arranged in a disordered manner. The lack of a clear consistency makes this alloy more malleable and ductile. The temperature limit is 460 degrees. However, it is worth cooling the brass below this indicator, as the strict ordering of the copper and brass atoms is restored. The harder the alloy, the more fragile it is.


The material finally melts at a temperature of 950 degrees, which puts it in the category of the least refractory. Due to its plasticity, brass can not only be turned, but also stamped at one of the characteristic stages of conveyor production.
The more zinc is in brass, the harder and more brittle the alloy becomes. However, the overall strength of brass is significantly inferior to steel. The presence of other metals and non-metals in brass affects the processing and ductility of the alloy. The properties achieved in this way are necessary for ease of turning and chip removal - not every product is made by casting.

Brass does not rust; parts made of it are used in conditions of high relative and absolute humidity of the environment. The characteristic properties inherent in copper products are manifested: in a relatively dry air, the thinnest oxide film that appears protects the layers lying deeper from decomposition, like a layer of paint. After grinding and turning, brass does not oxidize or blacken. However, it darkens in conditions of high humidity, in the presence of certain salts and acid fumes.
The alloy has fairly good varnish or paint coverage. This allows brass to acquire a truly marketable appearance - the buyer will not immediately guess what a particular part is made of.



The alloy has good anti-friction properties. Brass has high weldability with steel alloys and non-ferrous metals. It is easy to obtain, for example, bimetallic parts that are used in mechanics and electrical engineering.
The golden color - like bronze - is used in the production of interior luxury goods.
And brass almost does not magnetise due to the scanty content of iron and nickel: with the help of a hand magnet, even an experienced specialist in this regard cannot distinguish it from non-ferrous metals.


Composition
The percentage of zinc and copper in brass exceeds the amount of other components, which somewhat change the properties of this alloy. Copper gives brass additional ease of processing. There are two structures of brass.
- Alpha phase is a highly stable compound. The crystal lattice of brass, which has assumed the state of this phase, has a face-centered cubic shape. This alloy is the most common of the brass compositions.
- Alpha-beta phase - 3 parts copper and 2 parts zinc. The crystal lattice has elementary fragments.
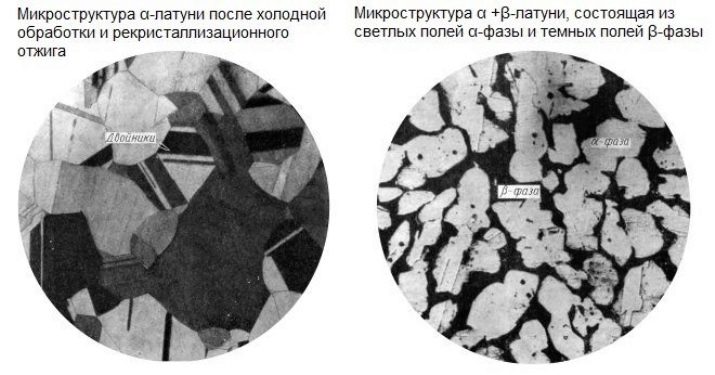
The hardness of the second phase is much higher than the first. But hardness and plasticity are mutually exclusive concepts. If the zinc in brass is about half, then the brass becomes almost white. The more zinc, the harder the brass alloy - copper gives the alloy greater softness and ductility.
The content of lead and bismuth in brass allows the processor to deform the product less when heated. Lead, introduced into the composition in a small amount, will make it possible to obtain easily crumbling sawdust, which makes it much easier to remove them from a newly cut edge.


The most widely used tombak is used in the production of parts and some jewelry. The color of the brass alloy in this case comes out yellow or reddish - by the color it is easy to determine how much zinc is used during smelting.
Views
Brass is mainly classified according to its chemical composition. The percentage of zinc, copper and other metallic and non-metallic additives largely determines its final physical parameters. So, almost white brass contains up to half of the zinc.
The highly deformable alloy contains about 88% copper and 10% zinc, the rest is additives. This is the so-called tombak - this modification has decent performance.


There is malleable brass that is used to make wrought iron and antiques. Some parts of it are plated with chrome or nickel - nickel-plated or chrome-plated brass looks more beautiful, since it does not outwardly lose its shade, given during the final processing.
The fluidity of brass upon heating and subsequent melting of the alloy makes it possible to cast objects with a high degree of detail.
In this case, milling of art objects is not required.


Jewelry brass is used for the production of pendants, rings, earrings and other jewelry. Brass can be coated with gold (gilding), which allows brass jewelry to be passed off as real gold, without overpaying tens and hundreds of times. This type of brass is used for the production of wrist watch cases - like jewelry, these watches can be gilded or silver plated.Before applying gold or silver, jewelry is pre-polished - polished brass shines from all sides, and precious metals will improve the appearance of jewelry to perfect condition.


Red brass contains 10% or less zinc. It is used for making figurines, small busts and other small sculptures.
In mechanics, foundry brass is used as moving and stationary parts of machines and devices. Due to its relatively low density - only 8.3 g / cm3 - it is used in functional units that benefit from lightening in order to improve driving performance. The alloy contains 50-81% copper, and the amount of third-party technological additives has been increased to 2-3%.


Parts made from foundry brass are used in machines and mechanisms of all kinds of technical devices, as well as in functional modules and blocks of modern ships and vessels. Casting alloy is the main component of valves: taps, gate valves, valves, for which the operating temperature does not exceed 250 degrees. Some bearings are made of brass rather than steel - mainly those that do not bear an increased load.
Automatic brass is used in precision mechanics. The copper content is 57-75% copper, zinc - 24-42%, lead - 0.3-0.8%. Automatic brass alloy is processed on high-precision and high-performance machines.


One of the technical alloys used for the production of hardware or elements for interior decoration has properties similar to automatic brass. Such blanks are in the form of rods and sheets. The former are machined on a lathe, the latter are milled and / or stamped.
Alpha alloy is characterized by a mass fraction of zinc not exceeding 35%. Due to the non-standard crystal lattice, which determines the internal structure, the alloy has considerable plasticity.
This modification of brass is ideal for stamping products.


Two-component
The brass alloy, containing mainly only copper and zinc, has only a small, trace quantitative content of other impurities. Pure two-component brass is a phenomenon that only occurs in laboratories. Zinc dissolves in copper at 20-25 degrees by 39%. When heated up to 950 °, when the alloy becomes liquid, the solubility of zinc in copper drops to 32%. Attempts to dissolve more zinc at the same 95 degrees will lead to the transition of brass from alpha to beta phase: excess zinc will either begin to precipitate or remain unevenly suspended, due to which the workpiece cast from beta brass will break at the very first serious mechanical (weight) load.


However, the behavior of brass with a gradual increase in the concentration of zinc in the alloy is not quite common and natural. As long as there is no more than 32% zinc in the alloy, the plasticity of the composition grows. But when passing through 32% at 950 degrees - and with subsequent solidification - fragility and hardness increase. After passing the 45% plank on zinc, the hardness and strength of the cast billet will drop sharply.
Brass works well with high pressure. But at 300-700 degrees, the alloy becomes unnecessarily brittle, and in this interval, brass is not processed in this way.

Cold working of a two-component alloy is carried out with a zinc content of up to 32%. This is how sheet, wire and profile blanks are obtained. At room temperature, this alloy is highly plastic. A decrease in plasticity at 300-700 degrees does not allow obtaining hot-rolled products - for such, the zinc content would need to be increased to 39%.
The marking of two-component brass is as follows. For example, L-80 is approximately 80% copper and 20% zinc. The marker number indicates the weight percent of copper in the alloy.


Multicomponent
Grades of multicomponent brass alloys have a larger number than grades of two-component alloys. In addition to copper and zinc, alloying is carried out using other components. The simple nomenclature assumes that brass, for example, supplemented with impurities based on iron and manganese, is called ferrous-manganese. Aluminum, for example, has a corresponding name.

Labeling of multicomponent formulations is more complex.
For example, LAZhMts66-6-3-2 contains 66% copper, 6% aluminum, 3% iron and 2% manganese. Zinc is present here in the amount of 23%. Zinc is not indicated in the name: it is calculated by the residue as a result of subtracting copper and alloying additives. In addition to iron, aluminum and manganese, silicon, lead and nickel are used as additives. When added in different percentages, they significantly change the properties of the alloy.
- So if manganese is added, the strength and resistance to oxidation of brass products increases markedly. Mixing with tin, aluminum and iron will increase this quality further.
- Thanks to tin not only will the strength increase, but also the resistance to oxidation in seawater. The fact is that this water contains salts, which under normal conditions would corrode iron and copper even faster than in an environment other than a maritime climate. Tin-containing brass is called "marine".
- Nickel It is distinguished by its ability to form an oxide film on any alloys that is resistant to destruction. This makes brass less susceptible to corrosion in high humidity environments.
- Lead facilitates processing, but deteriorates the strength of parts made of brass alloys. The malleability of brass with lead increases significantly. Its content in brass does not exceed 2% - this is how automatic brass is obtained, which received the name due to the fact that the production of parts and components is based on production using automated machines.
- Siliconwhile reducing strength and hardness, when combined with lead, it contributes to premature abrasion of bearing sets.
- Tin - individually - due to the antioxidant properties of brass in salt water, this alloy can be used in shipbuilding.
Brass shows good resistance in solutions of organic acids and salts based on them. The amount and percentage of alloying additions, with the exception of tin, has no additional effect on the alloy at this level.
How to distinguish from other metals?
Every employee of the scrap metal collection point knows how to distinguish brass from other non-ferrous metal alloys. If he does not possess this information, his work as a receiver can cause damage to enterprises that specialize in melting, processing of various secondary metals.
If even a schoolchild who does not have experience in the processing of metals and their alloys can distinguish brass from steel, then it is much more difficult to distinguish, for example, a brass alloy from bronze, steel with the addition of cobalt.


This is used by unscrupulous sellers, releasing, say, pure bronze and brass nuts instead of anodized steel nuts and bolts. The yellowish tint of brass depends on the zinc content and other additives in it. When you try to screw a self-tapping screw from a non-ferrous metal into a hole pre-drilled in a steel sheet or section of a profile, this fastener will simply roll to one side. When a brass self-tapping screw is screwed into a tree, the slot is easily damaged with a screwdriver or screwdriver's bat, and the element will definitely go to waste.


The difference between brass and copper is as follows. Copper is softer than brass - it is easily cut with wire cutters and metal scissors. Pure copper has the characteristic reddish color. The high copper content in brass, however, can confuse even the most experienced user.
- To understand that this is brass, not copper, throw the part on the ground or hit it with a hammer. Brass will make a ringing sound, while copper will make a dull sound. This check is necessary so as not to confuse identical massive parts containing kilograms of metal or alloy.
- Examine what kind of marker from the manufacturer (if any) is on the part. Brass is marked with the first letter L, and copper, respectively, M.
- If there is no identification mark, then try to scratch the product with a 10 or 50 kopeck coin. A significant, easily distinguishable groove will remain on the copper, which cannot be said about brass.
- Finally, make sure you are looking at a specific product. So, a string or electrical wires are made of copper. Brass can be furniture components, fittings for windows and doors, some dishes and part of tools, machine parts (for example, pipe adapters).



The differences from bronze are as follows.
- Brass is golden yellow, bronze is brownish red.
- Brass is lighter than bronze. Tin is significantly heavier than zinc - and it, in turn, is the second main component of bronze, along with copper. Bronze is considerably heavier than copper.
- Some bronze items are attracted by a magnet if the alloy contains a high content of iron and nickel.
- When exposed to an acid solution, a brass cup does not form a sediment, which cannot be said about bronze.
- Attempting to weld brass will result in whitish smoke. Bronze does not give such a reaction to the electric arc.


Experienced metalworkers can accurately identify brass and bronze by color by sharpening the product or its part with a file anywhere.
The difference between bronze and brass is too significant to be overlooked.


Applications
The composition of brass finally determines what exactly it is advisable to use to make.
- So, tombak with 90% copper is used for bi- and polymetallic products. A typical example is bimetallic plates in switches of electric kettles, which snap off independently at a steam temperature of more than 100 degrees of water that has begun to boil off.
- Gold-colored (decorative) brass, indistinguishable in appearance from the 595th sample of gold, is used to make earrings and chains, watch bracelets, etc. Jewelry is gilded or silver after molding. Tiles, elements of artistic forging, furniture components are anodized (for example, galvanized, chrome-plated, nickel-plated, etc.) or painted with varnish or paint of an unusual shade.
- The brass adapter can be welded to the steel tubing. However, it is difficult to weld these two parts with the simplest inverter - using conventional electrodes. More professional welding is used here. Applications for these adapters include gas and water supply, capillary tube systems, etc.
- Cast brass is used for the manufacture of load-bearing structures. This can be, for example, an W-shaped profile for sliding glass doors of furniture, however, it costs more than aluminum.
- Automatic brass is used for the production of fasteners, sheets and profiles. High-speed processing brings the production of these products to a truly massive level.
- Brass alloy is conductive like bronze. Brass is used for casing connections of wires and cables - for this, softer and more plastic grades of brass are used. Corrosion resistance is also important here - the contacts should not oxidize, leading to arcing under the load of the electrical line.


More specific areas of application for brass alloys of various grades:
- L96 - radiators, capillaries are made of this alloy;
- L8 / 85/90 - auto parts, components of climatic equipment;
- L70 - for casing chemical devices;
- L68 - stamping;
- L63 - fasteners, condenser tubes, auto parts;
- L60 - adapters, nuts, auto parts;
- LA77-2 - condenser pipelines of sea vessels;
- LAZH60-1-1 - details of ships;
- LAN59-3-2 - spare parts for ships, electric motors, chemical equipment;
- ЛЖМа59-1-1 - bearing cages, spare parts for aircraft and ships;
- LN65-5 - pressure gauges, condensers;
- LMts58-2 - fasteners, fittings, auto parts;
- LMtsA57-3-1 - spare parts for ships and floating crafts;
- L090-1 / L070-1 / L062-1 - storage tubes in heat engineering;
- L060-1 - condensers in heating engineering;
- LS63-3 / LS74-3 - watch parts, bushings;
- LS64-2 - spare parts for printing;
- LS60-1 - fasteners, gears of mechanisms, bushings.



Each type of brass alloy - out of dozens of known ones - has a specific application. It is not recommended to violate these requirements.








